Ẹ̀fúùfù abíire
(Àtúnjúwe láti Noble gas)
Àwọn Ẹ̀fúùfù abíire únjẹ́ lílò nínú kẹ́místrì láti fi ṣe ìsètò àwọn apilẹ̀ṣẹ̀ kẹ́míkà.
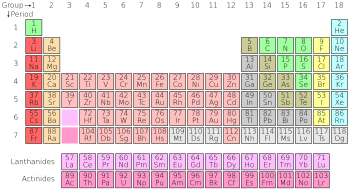
Ẹ̀fúùfù abíire
Halojín
Other nonmetals
Apart from hydrogen metallloids are not placed in the p-block.

|
Àyọkà yìí tàbí apá rẹ̀ únfẹ́ àtúnṣe sí. Ẹ le fẹ̀ jù báyìí lọ tàbí kí ẹ ṣàtúnṣe rẹ̀ lọ́nà tí yíò mu kúnrẹ́rẹ́. Ẹ ran Wikipedia lọ́wọ́ láti fẹ̀ẹ́ jù báyìí lọ. |
Itokasi
àtúnṣe- Bennett, Peter B.; Elliott, David H. (1998). The Physiology and Medicine of Diving. SPCK Publishing. ISBN 0-7020-2410-4.
- Bobrow Test Preparation Services (2007-12-05). CliffsAP Chemistry. CliffsNotes. ISBN 0-470-13500-X.
- Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- Harding, Charlie J.; Janes, Rob (2002). Elements of the P Block. Royal Society of Chemistry. ISBN 0-85404-690-9.
- Holloway, John H. (1968). Noble-Gas Chemistry. London: Methuen Publishing. ISBN 0-412-21100-9.
- Mendeleev, D. (1902–1903) (in Russian). Osnovy Khimii (The Principles of Chemistry) (7th ed.). http://www.archive.org/details/principlesofchem00menduoft.
- Ojima, Minoru; Podosek, Frank A. (2002). Noble Gas Geochemistry. Cambridge University Press. ISBN 0-521-80366-7. http://books.google.com/?id=CBM2LJDvRtgC.
- Weinhold, F.; Landis, C. (2005). Valency and bonding. Cambridge University Press. ISBN 0-521-83128-8.
- Scerri, Eric R. (2007). The Periodic Table, Its Story and Its Significance. Oxford University Press. ISBN 0-19-530573-6.
| Wikimedia Commons ní àwọn amóunmáwòrán bíbátan mọ́: Ẹ̀fúùfù abíire |